They are intended to relieve pain and restore the quality of life. If an implant turns out to have a flaw, however, the consequences can be dire – and not just for the patient. Serial losses that can rapidly spiral into the billions make it imperative that insurers exercise the utmost care in underwriting.
Implants are a growth market
In Germany alone, about 400,000 artificial hips and knees are implanted each year. A further 100,000 patients receive a cardiac pacemaker or a defibrillator. These types of surgical interventions will become even more common as further advances are made in materials and techniques, as populations become older and as the incidence of lifestyle diseases such as adipositas (obesity) rises. The use of implants is a great advance in improving the quality of life, particularly for elderly people. But interventions undertaken for purely aesthetic reasons are also a booming industry. More than 400,000 breast implants, including replacements of existing implants, are performed every year in the US. Only 20% of these are carried out to reconstruct the breast following illness or injury.
Not all operations proceed without complications and assure a future free of complaint. In addition to the unavoidable risks associated with any surgical intervention, product defects have frequently led to pain, the stress of additional treatment or even to replacement of the implant long before the end of its expected service life. The early failure of implants has given rise to costs exceeding US$ 100m for the indemnification of claims and/or product recalls. Prominent product liability cases have also led to lengthy discussions as to whether current licensing and certification procedures for implants still offer patients enough protection against product risks.
Implants are in the highest risk class
An implant has to be licensed or certified before it can be sold. From a regulatory standpoint, implants are classified not as medicinal products (drugs), but as medical devices and are therefore also governed by different laws and regulations. For example, there is frequently no requirement for clinical trials for this product group. Medical devices are normally classified in risk classes I, II or III, with class I comprising low-risk products such as dressings, walking aids and wheelchairs. Products categorised as class II are those deemed to entail moderate risk. Risk class III covers products entailing the highest risk, such as artificial hips and knees, cardiac pacemakers and breast implants. The higher the risk class, the tougher the licensing and certification requirements are.
The European certification procedure is organized in a decentralized way: The so-called Notified Bodies, which play an important role in this procedure operate in the private sector. Manufacturers can choose from about 80 Notified Bodies throughout the whole of Europe to have a product certified and given the CE marking. Since the Notified Bodies have a strong financial interest in engendering long-term customer loyalty, there is widespread criticism that certification procedures may be too lax. Clinical studies, including product tests on human beings, are frequently not included in the certification process. Attention focuses more on correct documentation. The product itself need not always be inspected. Major losses in connection with faulty breast implants and defective hip implants have frequently hit the headlines and in 2016 led to the adoption of a new European Regulation on Medical Devices, which does not fundamentally change the existing certification system but aims to increase the safety of implants through additional regulatory requirements.
In the US, the licensing of medical products is regulated by a federal government agency, the Food and Drug Administration (FDA). There are two different processes: “Premarket Approval” (PMA) and “Premarket Notification”, also referred to as the 510(k). A PMA is required for products classified as entailing the greatest risk, class III, which includes knee and hip implants. Just as in the process for approving drugs, a product's safety and efficacy must be demonstrated in clinical trials in order for it to obtain approval. According to a Supreme Court ruling, such a PMA constitutes a “mark of quality” and reduces the manufacturer's risk of being sued in product liability actions.
Loopholes instead of clinical studies
Intended for medical products entailing relatively low risk, the 510(k) procedure is considerably less complex and generally only requires proof that the new product under consideration is much the same as a previous product. Clinical trials are not required. However, rules governing phases of transition from one law to another also allow this simplified procedure to be applied to a number of products in the highest risk class, III. As a result, manufacturers can refer to an “original product” from before 1976, for which no proof of safety or efficacy was ever required. Particularly for products in risk class III, this created a loophole through which manufacturers could dispense with complex clinical trials and market particularly high-risk products without any adequate proof of safety. Manufacturers of “510(k) implants” run a distinctly higher risk that product liability claims may be filed against them, as they lack the FDA's “mark of quality”, namely the PMA.
Quality and risk management
The main factors in reducing the product liability risk posed by implants are for the manufacturer to institute and practise professional-standard quality management and risk management. Both can be prescribed by law. Quality management must take account of more than just the product's safety requirements, as testing product safety involves more than simply checking the quality. These tests also include assessing the product's susceptibility to faults, their effects and the product's inherent safety. Such risk management aspects are very important in product liability insurance and should certainly be taken into account in underwriting. Misassessing a product's risks may prove expensive for an insurer and could even mean bankruptcy for the manufacturer.
ISO 14971, an international standard for medical products, offers a risk management guideline. In addition to describing the basic requirements, it covers all relevant aspects of risk management, such as risk analysis, risk assessment, controlling risks, acceptance of the residual risk and reporting. Corresponding documentation should be available to underwriters for detailed examination. In case of doubt, they should not rely solely on this documentation, but consider an on-site analysis of the risk.
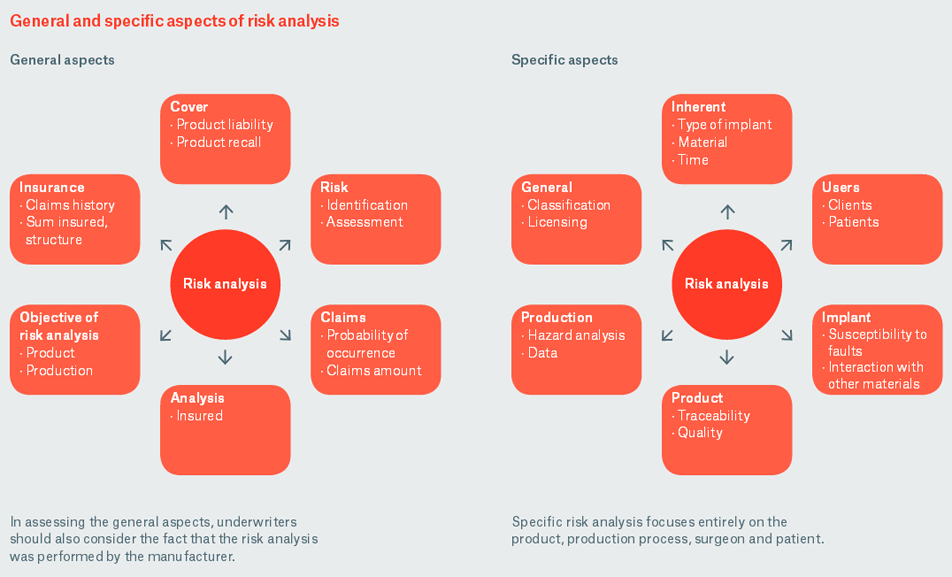
In analysing a risk, one can distinguish between the general aspects that describe the basic conditions and an individual analysis that focuses much more strongly on the product itself, its production and its use.
A product with the potential for major loss
Implants occupy a special position in product liability insurance. They entail high loss potential and must therefore fulfil especially high quality standards. These risks confront not only large multinational manufacturers, but also the great many small and medium-sized firms operating in this field: Continual product optimisation, the use of new materials and efforts to provide solutions customised to individual patients pose substantial development risks. In the event of a claim, it can prove difficult to distinguish between product liability and the surgeon's professional liability. Was the product really defective or may the surgeon have worked negligently? Was the surgeon not adequately instructed by the manufacturer of the implant?
Ultimately, “removing” an implant because the product has been recalled means additional time in hospital for the patient and normally involves considerably higher costs than when recalling and removing technical parts in the automotive industry, for example. High claims for damages can become an incalculable burden for the manufacturer.
Risk monitoring at Munich Re
As our experts constantly monitor the development of implant risks, they are able to share their know-how with their clients and provide valuable tips on risk-commensurate underwriting, which is absolutely essential in this field.